FDA / CMS / STUDIES
We are approved for studies with all of the major reading centers, including, but not limited to:
FPRC – University of Wisconsin, Madison – Fundus Photograph Reading Center
DARC – New York – The Digital Angiography Reading Center
Duke University Fundus Reading Center
DIRC – Doheny Image Reading Center
DRCRnet – Diabetic Retinopathy Clinical Research Network
Images for reading centers may be exported as jpg or png format,
and as anonymous for either.
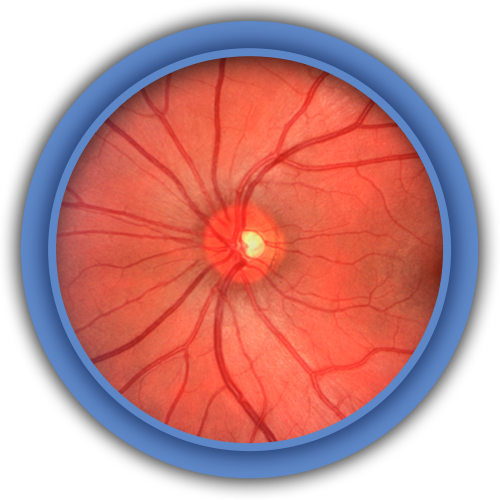
Fundus Photo has US FDA 510(k) clearance.
We are the only ophthalmic digital imaging manufacturer in this segment to achieve the highest level of quality, safety and performance the US FDA, CE and ISO require.
Did you know…
Medicare requires FDA approved devices for payment and FDA reports all device approvals to CMS?
All devices, attachments or software used for diagnosis and/or treatment are required to have FDA clearance for commercial distribution in the US.
Medicare Benefit Policy Manual, Chapter 14 – Medical Devices, sections 10 & 80 https://www.cms.gov/manuals/
Devices that may be covered under Medicare include the following categories:• Devices approved by the FDA through the Pre-Market Approval (PMA) process;
• Devices cleared by the FDA through the 510(k) process;
• FDA–approved IDE Category B devices; and
• Hospital Institutional Review Board (IRB) approved IDE devices
Look up any company at the FDA website http://www.accessdata.fda.gov/
Topcon, Zeiss, Escalon, Kowa, Nidek, Canon and Fundus Photo all have FDA clearance and may legally market their imaging products (hardware and software) in the US.
Fundus Photo unveils it’s low price digital imaging solution for mydriatic fundus cameras.
New Vision Ophthalmic Medical Imaging Software…
Fundus Photo is the exclusive dealer for North America, South America and Central America